Table of Contents
- 1 What is a biomarker?
- 2 Urine biomarker tests for bladder cancer
- 3 Urine test systems
- 4 Urine biomarker tests
- 4.1 Nuclear matrix protein 22 (NMP22; NMP22®, BladderChek ®) (1)
- 4.2 Bladder tumour antigen (BTA; BTA stat ®, BTA TRAK®)
- 4.3 Fluorescence in situ hybridisation (FISH; UroVysionTM) (5)
- 4.4 Cytokeratin fragment test (UBC® Rapid/Elisa)
- 4.5 Multigene RNA test (Cxbladder®)
- 4.6 Epigentic Profile test (AssureMDx TM)
- 4.7 Morpho-histochemical staining test (CellDetect®)
- 5 Available biomarker test characteristics
- 6 Questions to ask your doctor
- 7 References
What is a biomarker?
Biomarkers are substances that doctors can measure in the body to help them tell if a patient has a disease, how a disease is developing or if a treatment is working.
Urine biomarker tests for bladder cancer
Early detection of bladder cancer can improve the chance of successful treatment. Therefore, doctors try to find urine biomarker tests, which will help to identify bladder cancer or recurrence of bladder cancer as early as possible.
Your doctor may recommend the use of urine biomarker tests, if you are considered a high-risk patient (for example if you are or were a heavy smoker, have or had occupational exposure to certain substances or because of your family history) or bladder cancer is suspected from signs and symptoms, other laboratory tests or physical examination. In case of a urine biomarker test that gives a positive result, further examinations (for example a cystoscopy) are indicated to rule out or confirm bladder cancer.
Some doctors find these urine tests useful in looking for bladder cancers, but they may not help in all cases. Most doctors feel that cystoscopy is still the best way to find bladder cancer. Some of these tests are more helpful when looking for a possible recurrence of bladder cancer in someone who has already had it, rather than finding it in the first place.
In the following section, different test systems your doctor may use are introduced. You may use this information when consulting your doctor, and when considering why an additional test may or may not be useful for you.
Urine test systems
Some tests may not be available in your country. Ask your doctor about the availability of urine biomarker tests in your country and the manufacturer’s website address for more information.
Urine dipstick analysis
Using a urine dipstick analysis is an easy method for the detection of blood in the urine not visible to the eye (microhaematuria). In most cases, the reason for blood in the urine has benign causes (like infections), but 5% to 10% of patients with blood in the urine have urinary tract cancer. Therefore, checking for blood in the urine may help in early bladder cancer detection. If bladder cancer as a reason cannot be excluded, you should consult a urologist for further examinations.
Urine cytology
In this test, a specially trained expert evaluates the cells in the urine under a microscope after special staining of the cells. This test can reliably detect aggressive cancer cells (high grade), but benign looking cancer cells (low grade) are more difficult to identify since benign conditions such as calculi, inflammation or infections can lead to changes in the cells imitating cancer.
Urine biomarker tests
Nuclear matrix protein 22 (NMP22; NMP22®, BladderChek ®) (1)
This test uses the detection of NMP22, a protein which can be found in the nucleus of the main command centre of our cells that takes care of important cell regulatory activities. It’s also the main place where our DNA is stored. NMP22 is thought to be released from disintegrating cells and elevated NMP22 levels in the urine can be found in bladder cancer patients. The test can be performed and analysed in the doctor’s practice as a point-of-care test or in a specialised laboratory (5-7).
Bladder tumour antigen (BTA; BTA stat ®, BTA TRAK®)
This test uses the detection of BTA (human complement factor H related protein) in the urine. BTA can be produced by human bladder cancer cells and enhances the degradation of certain parts of our immune system. The test can be performed and analysed in the doctor’s practice as a rapid test (point-of-care test) or in a specialised laboratory (7, 8).
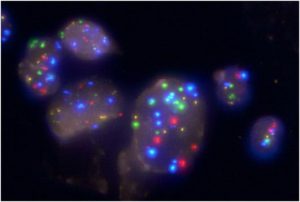
Fluorescence in situ hybridisation (FISH; UroVysionTM) (5)
In this test, common genetic changes of bladder cancer cells (involving chromosomes 3, 7, 17 and 9p21) in the urine are analysed in a specialised laboratory. As in urine cytology, a specially trained expert evaluates the appearance of the cells in the urine. Additionally, the genetic areas of interest are marked by a fluorescent dye (Fig. 1), making them visible for analysis using a special microscope (fluorescence microscope). The pattern of genetic signals obtained with the test in relation to other cytological characteristics can help the expert with the diagnosis (7, 9, 10).
Cytokeratin fragment test (UBC® Rapid/Elisa)
In this test, fragments of special cell components (in this case specific parts of the cytoskeleton, i.e. cytokeratin 8 and 18) in the urine are measured. The test can be performed and analysed in the doctor’s practice as a point-of-care test or in a specialised laboratory (10-12).
Multigene RNA test (Cxbladder®)
In this test, five special gene biomarkers (CDC2, HOXA13, MDK, IGFBP5, and CXCR5) are analysed from your urine in a specialised laboratory. Your doctor will get a report, in which a bladder cancer risk score is calculated as a result of the analysis of the five biomarkers (13).
Epigentic Profile test (AssureMDx TM)
In this test, the methylation status of three genes (TWIST1, ONECUT2 and OTX1) in combination with mutation analyses of three others (FGFR3, TERT and HRAS) is analysed. The test is designed to provide evidence regarding the presence or absence of bladder cancer and possible prognostic information. The test is performed by a specialised laboratory (14, 15).
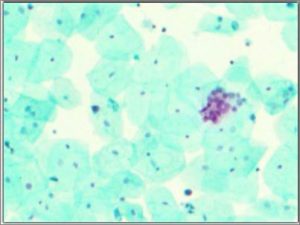
Morpho-histochemical staining test (CellDetect®)
In this test, cells from the urine are stained with a certain plant extract and three dyes in a specialised laboratory and analysed by a specially trained expert under a microscope. The test produces a green-blue staining in normal cells and a pink-red staining in tumour cells (Fig. 2), which can be interpreted in relation to the cell morphology like in cytology, which means that the expert can evaluate the appearance of the cells in cytology and gets additional information from the test’s staining results of the cells (16).
Available biomarker test characteristics
Table 1: Available biomarker test characteristics.
| Biomarker/test system | Overall sensitivity (%) | Overall specificity (%) |
| FISH (UroVysionTM) | 30-86 | 63-95 |
| NMP22 | 47-100 | 55-98 |
| BTA stat ® | 29-83 | 56-86 |
| BTA TRAK® | 53-91 | 28-83 |
The table and information is taken from the EAU Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1 and CIS) – Update 2016 [1]. It includes biomarker test systems reviewed there.
For the meaning of sensitivity (true positive rate of a test) and specificity (true negative rate of a test).
Values like sensitivity and specificity of tests are listed in percentages: 0-100%.
An ideal test with 100% sensitivity and specificity would correctly identify all patients with the disease, and similarly, correctly identify all patients who are disease-free. Most clinical tests fall short of this ideal.
An example:
Sensitivity
A test with 100% sensitivity correctly identifies all patients with the disease. A test with 80% sensitivity detects 80% of patients with the disease (true positives) but 20% with the disease go undetected (false negatives).
Specificity
A test with 100% specificity correctly identifies all patients without the disease. A test with 80% specificity correctly reports 80% of patients without the disease as test negative (true negatives) but 20% patients without the disease are incorrectly identified as test positive (false positives).
This means that in the case of a biomarker test that gives a positive result, further examinations (like imaging or biopsy) are indicated to rule out or confirm the disease.
Questions to ask your doctor
- What is a urine biomarker?
- Is a urine biomarker test indicated for me?
- If a urine biomarker test is indicated, which one best suits my situation?
- What does a positive or negative urine biomarker test result mean?
- How reliable are urine biomarker test results in general and the test that was used in particular?
- Can a urine biomarker test replace other invasive examinations?
References
- Babjuk M, et al. EAU Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1 and CIS) – Update 2016: European Association of Urology, 2016.
- Hall MC, et al. Guideline for the Management of Nonmuscle Invasive Bladder Cancer: Stages Ta, T1 and Tis: Update (2007): American Urological Association, 2014.
- Witjes JA, et al. EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer – Update 2016: European Association of Urology, 2016.
- van Rhijn BWet al. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol 2005;47:736-748.
- Soloway MS, et al. Use of a new tumor marker, urinary NMP22, in the detection of occult or rapidly recurring transitional cell carcinoma of the urinary tract following surgical treatment. J Urol 1996;156:363-367.
- IGeL Monitor: NMP22-Test zur Früherkennung von Harnblasenkrebs. In: MDS: Medizinischer Dienst des Spitzenverbandes Bund der Krankenkassen; 2014.
- Chou R, et al. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:922-931.
Malkowicz SB. The application of human complement factor H-related protein (BTA TRAK) in monitoring patients with bladder cancer. Urol Clin North Am 2000;27:63-73, ix. - Veeramachaneni R, et al. Evaluation of fluorescence in situ hybridization as an ancillary tool to urine cytology in diagnosing urothelial carcinoma. Diagn Cytopathol 2003;28:301-307.
- Tilki D, et al. Urine markers for detection and surveillance of non-muscle-invasive bladder cancer. Eur Urol 2011;60:484-492.
- Sanchez-Carbayo M, et al. Initial evaluation of the diagnostic performance of the new urinary bladder cancer antigen test as a tumor marker for transitional cell carcinoma of the bladder. J Urol 1999;161:1110-1115.
- Ritter R, et al. Evaluation of a new quantitative point-of-care test platform for urine-based detection of bladder cancer. Urol Oncol 2014;32:337-344.
O’Sullivan P, et al. A multigene urine test for the detection and stratification of bladder cancer in patients presenting with hematuria. J Urol 2012;188:741-747. - Millholland JM, et al. Detection of low frequency FGFR3 mutations in the urine of bladder cancer patients using next-generation deep sequencing. Res Rep Urol 2012;4:33-40.
- Karnes RJ, et al. A noninvasive multianalyte urine-based diagnostic assay for urothelial cancer of the bladder in the evaluation of hematuria. Mayo Clin Proc 2012;87:835-842.
- Davis N, et al. A novel urine cytology stain for the detection and monitoring of bladder cancer. J Urol 2014;192:1628-1632.